Data Management
Ensuring integrity, accessibility, and compliance in the handling of project data.
Guiding Principles
DOPA-Kiss adheres to the highest standards of research integrity, implementing rigorous protocols for data collection, storage, and analysis.
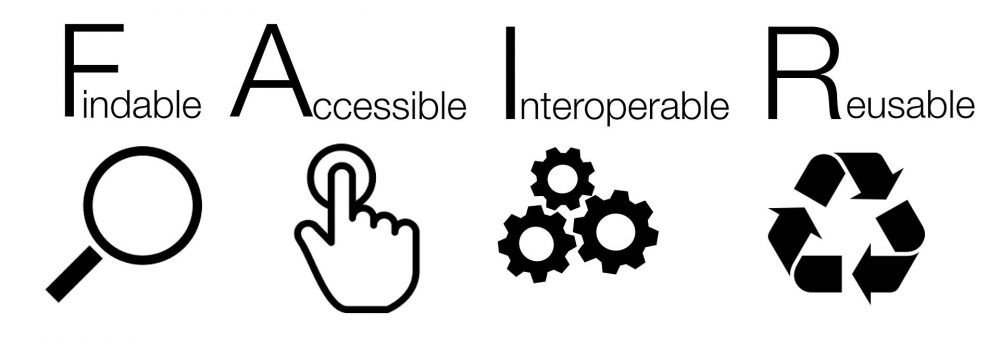
The project’s Data Management Plan (DMP) is designed in accordance with the FAIR principles—ensuring that all data are Findable, Accessible, Interoperable, and Reusable—while fully respecting ethical, legal, and confidentiality requirements.
Data Lifecycle
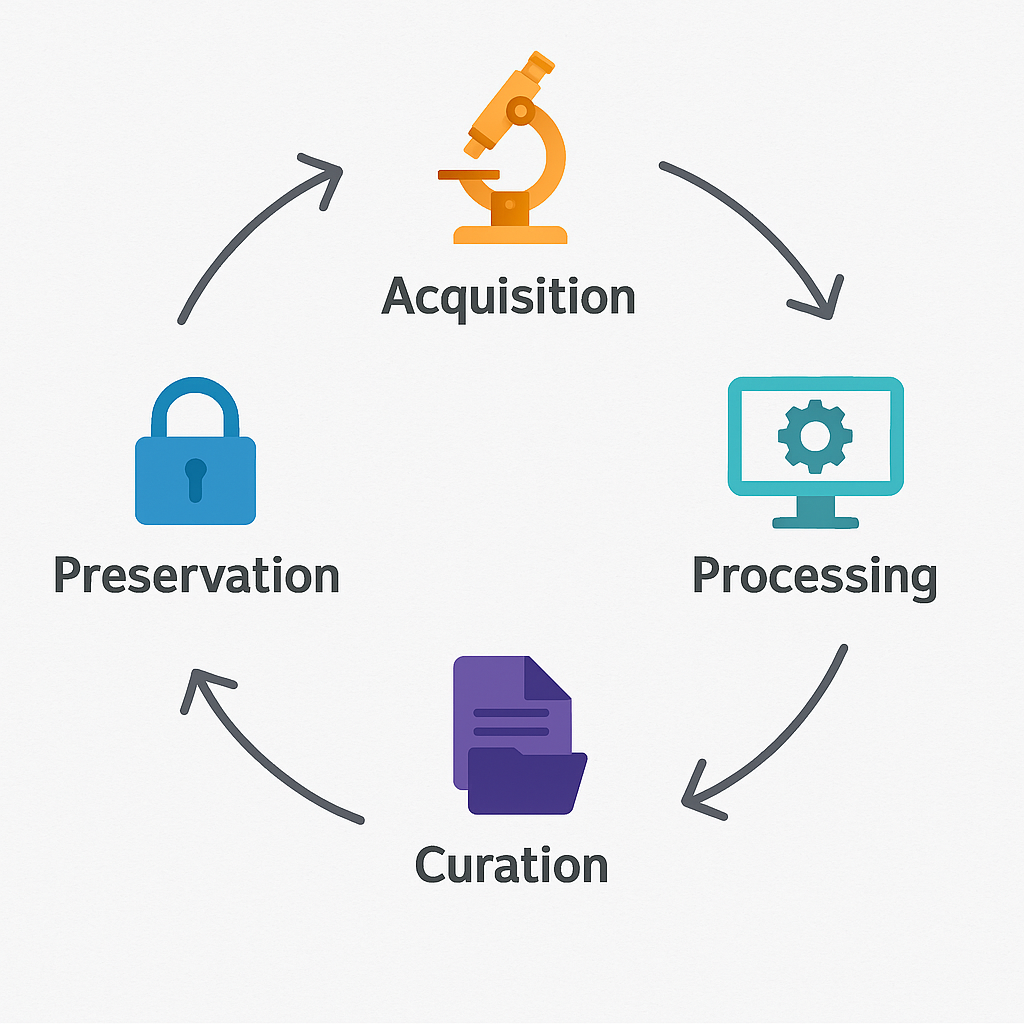
The project’s data management framework spans the entire research lifecycle:
✅ Acquisition: Data generated through experimental work, imaging, multi-omics analyses, and computational models are collected using validated protocols.
✅ Processing and Storage: All raw and processed datasets are stored in secure institutional repositories, with controlled access for authorised team members.
✅ Curation and Documentation: Comprehensive metadata accompany all datasets to enable reproducibility and long-term usability.
✅ Preservation and Sharing: Data selected for public release are deposited in open repositories, following embargo periods when required.
Ethical and Legal Compliance
All data management activities are conducted in strict adherence to relevant regulations, including the General Data Protection Regulation (GDPR) for personal data, and applicable national and institutional policies.
Sensitive or identifiable data are anonymised or pseudonymised prior to storage and sharing.



Open Science and Dissemination
Consistent with the principles of open science, DOPA-Kiss promotes the publication of datasets in open-access repositories whenever possible.
Publicly available datasets will be linked from the project website, ensuring visibility and facilitating secondary use by the scientific community.